The Energy Needed to Start a Chemical Reaction Is Called
The Activation Energy The requirements for a chemical reaction are A collision The correct orientation of collision The required amount of energy activation energy to happen. All chemical reactions involve energy.

Chemical Kinetics Definition Equations Facts Britannica
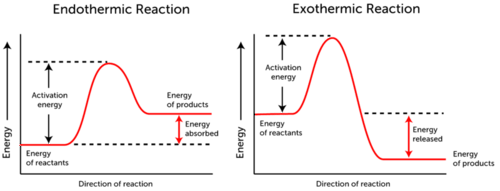
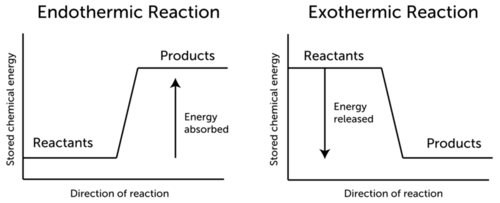
However no all chemical reactions release energy as rusting and also burning do.

. Activation energy is the minimum energy required to cause a reaction to occur. ExplanationThe energy required is called the activation energy. The initial energy needed to start a chemical.
The energy required to begin a reaction is known as activation energy. Activation energy is called the energy required to initiate a chemical reaction. The energy needed to destabilize existing chemical bonds and start a chemical reaction is called.
Instead they hasten reactions that would occur eventually The reaction catalyzed by each enzyme is very. The energy required to start a reaction is called _____. This kind of reaction can be stood for by a basic chemical equation.
All chemical reactions even exothermicreactions need a certain amount of energy to get started. ExplanationThe energy required is called the activation energy. Even reactions that release energy need a boost of energy in order to begin.
The energy necessary to start a chemical reaction. The energy required to start a chemical reaction is called. This energy is called activation energy.
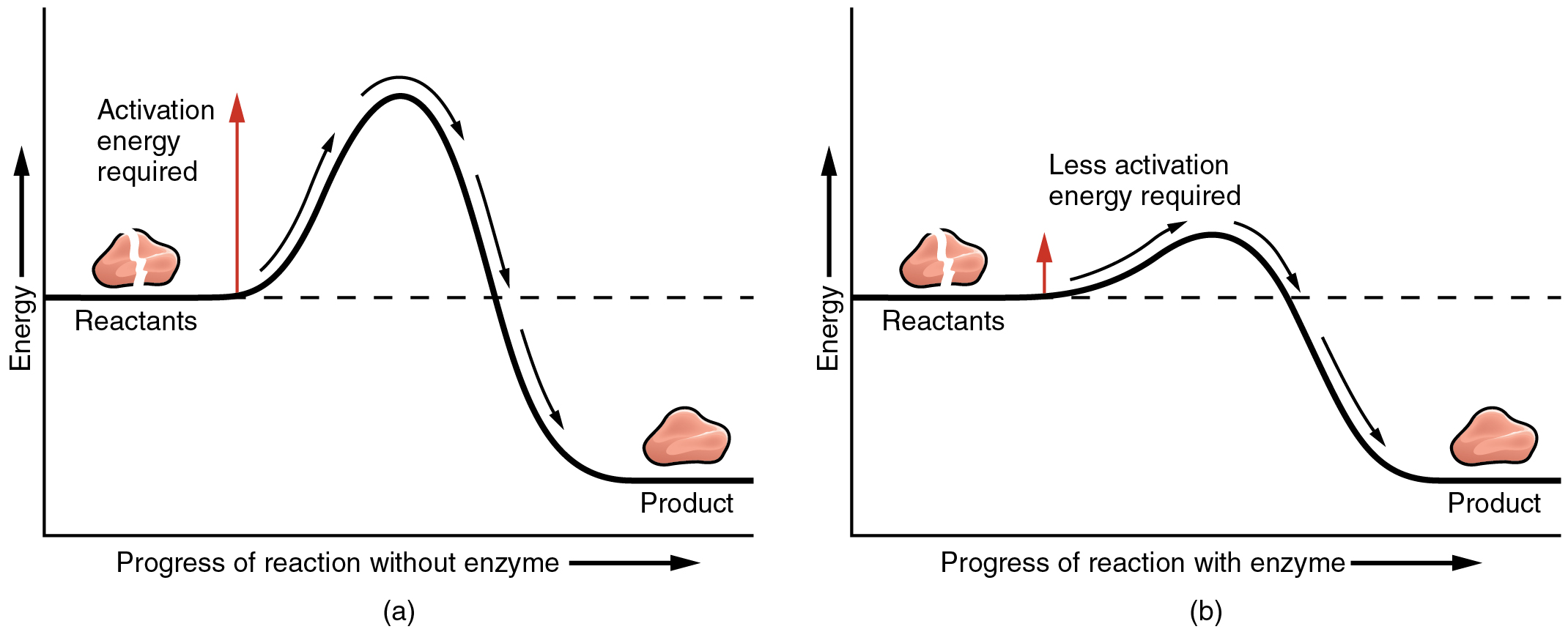
The energy required to begin a reaction is known as activation energy. The initial energy needed to start a chemical reaction is called the free energy of activation or activation energy EA Activation energy is often supplied in the form of thermal energy that the reactant molecules absorb from their surroundings Enzymes catalyze reactions by lowering the EA barrier Enzymes do not affect. For example activation energy is needed to start a car.
In some chemical reactions power is absorbed rather 보다 released. O speed up slow down cannot be determined remain unchanged. The energy needed to start a chemical reaction in the body is called and is raised or lowered by enzymes.
The bond energy of each carbon-oxygen bond in carbon. All chemical reactions even exothermic reactions need a certain amount of energy to get started. The energy needed to start a chemical reaction is called the activation energy.
Chemical reactions need a certain amount of energy to begin working. A protein that supplies water for hydrolysis reactions C. Still more energy is needed to start breaking bonds in reactants.
The energy is used in order to break the reactants chemical bonds. The energy needed to start a chemical reaction is called activation energy. 2CO O2 2CO2 Carbon monoxide and oxygen combine to produce carbon dioxide.
Then the atoms form the products new bonds. The minimum amount of energy needed to start a chemical reaction is given by the formula Ea-RTlnkA. For example activation energy is needed to start a car.
Question 11 1 pts If the shape of an enzymes active site were to change the rate of the reaction it catalyzes would. To understand activation energy we must first think about how a chemical reaction occurs. 1- Use the reaction to complete the sentence.
The energy needed to destabilize existing chemical bonds and start a chemical reaction is called asked Aug 25 2019 in Biology Microbiology by JAguilar559 A. The push gives the child enough energy to start moving but once she starts she keeps moving without being pushed again. The most common form of activation energy is going to be heat.
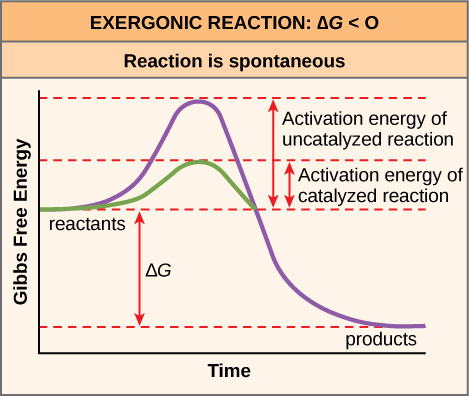
A chemical reaction the releases energy is called an exergonic reaction. The energy needed to start a chemical reaction is called 1 potential energy 2 kinetic energy 3 activation energy 4 ionization energy. The total bond energy of the products is 1472 kJ.
A protein that facilitates a reaction B. The initial energy needed to start a chemical reaction is called the free energy of activation or activation energy E A In catalysis enzymes or other catalysts speed up specific reactions by lowering the E A barrier Enzymes do not affect the change in free energy Δ G. Catalytic energy What is an enzyme.
A protein that absorbs water during dehydration reactions The prokaryotic. What is the energy that is required for a chemical reaction to start called. In bespeak to evaluate the means energy operation into and out of biological systems that is essential to understand much more about the various energy types that exist in the physical world.
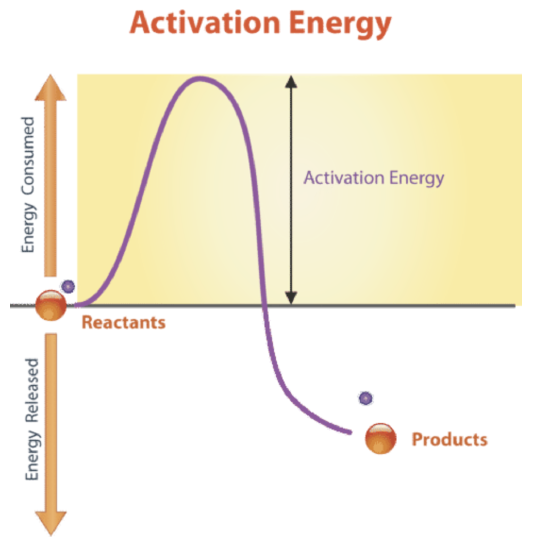
This energy is called activation energy. The minimum amount of energy required to initiate a chemical reaction is Activation Energy. The most common form of activation energy is going to be heat.
The energy necessary to initiate a chemical reaction is called activation energy. The minimum amount of energy needed to start a chemical reaction is given by the formula Ea-RTlnkA. The smallest amount of energy needed to start a chemical reaction is called temperature concentration surface area catalyst what four factors affect how.
Activation energy is like the push a child needs to start going down a playground slide.

18 4 Potential Energy Diagrams Chemistry Libretexts

What Is The Minimum Amount Of Energy Required To Start A Chemical Reaction

Bond Energy Definition Illustration Solved Problems Problem Solving Solving Covalent Bonding

Heat Of Combustion Physical Chemistry Chemistry Chemical Equation
Chemical Reactions Anatomy And Physiology

Activation Energy Article Khan Academy

Potential Energy Diagrams Chemistry Catalyst Endothermic Exothermic Reactions Youtube
3 9 Energy In Chemical Reactions Biology Libretexts

Pin By The Project Artist On Understanding Science In 2022 Chemical Reactions Molecules Understanding

Heat Of Reaction Chemistry Britannica
6 2d Activation Energy Biology Libretexts

Oxygen Shells Oxygen Chemistry Projects Binding Energy

How Enzymes Speed Up The Chemical Reactions Ck 12 Foundation

Activation Energy Definition Formula Si Units Examples Calculation
Activation Energy Ck 12 Foundation

You Need To Understand The Difference Between Endergonic And Exergonic Teaching Chemistry Biology Resources Chemistry

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy

Activation Energy Article Khan Academy
Conservation Of Energy In Chemical Reactions Ck 12 Foundation
Comments
Post a Comment